- What is a Pap Smear and Why is it Performed?
- Who Should Receive a Pap Smear?
- What are the Latest Statistics on Pap Smears and Cervical Cancer?
- What is the Human Papillomavirus (HPV)?
- How Should a Woman Prepare for a Pap Smear?
- How is a Pap Smear Performed?
- Are There Any Risks to a Pap Smear?
- How are Cervical Cells Evaluated?
- How are the Results of a Pap Smear Described?
- What are the Latest Advances with Pap Smear?
- What Can Affect the Results of a Pap Smear?
- What Additional Tests May Be Ordered if the Results of a Pap Smear are Abnormal?
- Additional Resources and References
Recently, new techniques have been developed to further improve cervical cancer cell sample collection and specimen quality. Hologic’s ThinPrep System and MediSpectra, Inc.’s LUMA Cervical Imaging System are two such new techniques that have been approved by the U.S. Food and Drug Administration (FDA). While the conventional Pap smear is still an accurate method of detecting abnormalities or cancer, research has shown that these new techniques may be more effective at detecting cervical cancer and pre-cancerous conditions than the conventional Pap smear by:
- making the patient’s slide more representative of the patient’s clinical condition
- improving the preservation of the sample
- standardizing the presentation of cells on the slide
- reducing mucus, blood, or other debris that may eclipse pre-cancerous or cancerous cells
A 1991 study of 600 laboratories found that up to 20% of Pap smear slides are unsatisfactory for evaluation and 40% are satisfactory but of limited value. Reasons for these classifications include too few cells to evaluate or too much mucus or blood in the sample to make an accurate interpretation.
With the conventional Pap smear technique, cervical cells are collected with a small stick or spatula and smeared on a slide for pathological analysis. However, a 1994 study published in the American Journal of Clinical Pathology found that up to 80% of a sample taken from a patient using the conventional Pap smear technique is not smeared on the slide but remains on the collection device. Instead of smearing the cervical cells on a slide after they are removed from the patient, new "direct-to-vial" techniques involve immediately rinsing the collected cells in a vial filled with a special solution. This reduces the likelihood that a patient’s cell sample will be damaged by air, clumping, etc.
The vial is then taken to the laboratory for slide preparation and screening. In the laboratory, the vial is inserted in a sample preparation device which breaks up blood, mucus, and other problematic materials. The thin layer of cells in then transferred to a slide and is automatically deposited into a preservative solution. With these newer methods, physicians are also able to conduct multiple analyses (such as HPV testing) using residual cells collected in the vial instead of having to order an additional Pap smear.
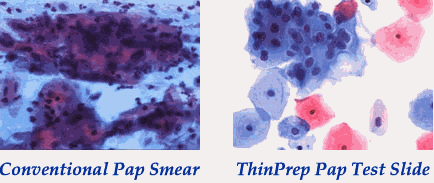 |
With conventional Pap smear, cells can be obscured by blood, mucus or clumping. With direct-to-vial techniques such as the ThinPrep method, more cells are preserved and there is less overlapping, blood, mucus, etc. Images courtesy of Hologic/Cytyc. |
In a clinical trial of 6,747 patients conducted by Cytyc, the maker of the ThinPrep direct-to-vial technique, researchers found a 65% improvement in the detection of cervical cancer at three screening centers using this new technique and a 6% improvement at three hospitals where the incidence of cervical cancer is historically high. The direct-to-vial technique was also found to be more effective at detecting severe cervical lesions than the conventional Pap smear. In all, more than 30 major studies including more than 300,000 patients in the United States, Europe, Asia, Africa, and Australia have found that "direct to vial" Pap smear techniques have benefits over the conventional cervical cell collection.
While new direct-to-vial methods have been shown to be more effective at detecting cervical cancer than the conventional Pap smear, the cost of these new methods is higher. Some insurance companies do provide coverage for these newer techniques while others do not. Therefore, women should check with their insurance companies prior to choosing these newer techniques. In some cases, women who choose to have the new direct-to-vial sample collection will have to pay out-of-pocket for the additional cost of the test.
Another advance in Pap smear screening is the use of computerized instruments that can recognize abnormal cells in Pap smears (similar to the use of computer-aided detection with mammography). An example of this technology is the AutoPap system made by Tripath Imaging, Inc. Normally, technologists and physicians evaluate all Pap smear samples. However, with this technology, a computer re-examines the sample and marks areas of the sample that may indicate abnormal cells. The technologist or physician then takes a closer look at these areas. The advantage of this technology is that the computer instruments may find pre-cancerous or cancerous cells that a technologist or physician may miss. However, some physicians believe that the technology can lead to a significant number of "false positive" results (the technology falsely indicates that the sample contains abnormal cells). These false positive results can lead to unnecessary repeat Pap smears, colposcopy, or other exams. As with the "direct to vial" techniques, this method may or may not be covered by insurance. Nevertheless, with continued improvements, many physicians believe this type of technology will eventually lead to more accurate detection of cervical cancer and pre-cancerous conditions.
Menstrual blood, vaginal lubricants, douches, or vaginal medications may cause inaccurate results of a Pap smear. Also, failure to apply a preservative to the slide sample immediately after cervical cells are obtained and spread onto the slide may cause the cells to become dried out.
- Colposcopy: The cervix is viewed through a colposcope (an instrument with magnifying lenses) to check for abnormalities.
- Cervical biopsy: A portion of tissue from the cervix may be removed for further examination and to confirm if cancer is present.
- Cone biopsy: An elaborate cervical biopsy, a cone biopsy involves removing a cone-shaped region of tissue high on the cervical (that would not be seen with a colposcopy).
Click here to learn more about how cervical cancer is diagnosed.
- The National Cancer Institute provides information on Pap smears at http://cancernet.nci.nih.gov/
- The American Cancer Society provides information on Pap smears at http://www3.cancer.org/cancerinfo/load_cont.asp?ct=8&doc=26
Updated: November 21, 2007



